An embryo-fetal development toxicity study with dimethylaminoethyl ginkgolide B in rats and rabbits
Abstract
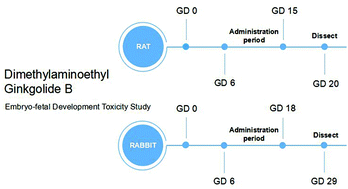
Ginkgo biloba (a herbal product) has long been used in traditional Chinese medicine to treat cerebrovascular and cardiovascular diseases. Ginkgolide B is one of the important pharmacologically active components of Ginkgo biloba. Dimethylaminoethyl ginkgolide B (a novel ginkgolide B derivative) has been developed to overcome the poor water-solubility of natural drugs and to achieve higher bioavailability. This study investigated the potential effects of dimethylaminoethyl ginkgolide B on pregnant dams and embryo-fetal development in Sprague-Dawley rats and New Zealand white rabbits following maternal exposure on gestational day (GD) 6–15 and GD 6–18, respectively. Dimethylaminoethyl ginkgolide B was administered by intravenous injection to pregnant rats (0, 10, 30 and 100 mg kg−1 d−1) and rabbits (0, 6, 18 and 60 mg kg−1 d−1). Maternal toxicity signs, such as lower maternal body weight gain and food consumption, were observed at 100 mg kg−1 d−1 in rats and 60 mg kg−1 d−1 in rabbits. The developmental toxic effects included a decrease in fetal and placental weights, increased incidences of skeletal variations and delay in fetal ossification. Fetal growth and development were affected by dimethylaminoethyl ginkgolide B in the high-dose group in rabbits. The no-observed-adverse-effect level of dimethylaminoethyl ginkgolide B is considered to be 30 mg kg−1 d−1 for rats and 18 mg kg−1 d−1 for rabbits.


 Please wait while we load your content...
Please wait while we load your content...