A pure red luminescent β-carboline-substituted biphenylmethyl radical: photophysics, stability and OLEDs†
Abstract
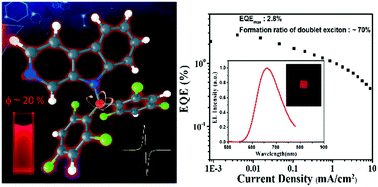
Luminescent radicals, whose emission comes from the doublet exited state, have potential application in the field of organic optoelectronics. However, few radicals show luminescence at room temperature. Herein, a new pure red-emissive biphenyl-type (N-pyrido[3,4-b]indolyl)bis(2,4,6-trichlorophenyl)methyl radical (PyID-BTM) is designed and synthesized, in which the carbazole moiety of CzBTM (a previously reported biphenylmethyl radical by our group) is successfully replaced by β-carboline. Its photophysical properties, including the ground and excited states, and the radiative and non-radiative processes are systematically investigated. The photoluminescence quantum efficiency (ϕ = 19.5%) of PyID-BTM is ten times higher than that of CzBTM (ϕ = 2.0%) in cyclohexane. The crystal structure, magnetic properties, photostability and electroluminescence performance of PyID-BTM are studied. An optimized OLED device using PyID-BTM as an emissive dopant showed pure red emission with CIE coordinates of (0.649,0.317) and a maximum EQE of 2.8%, and the formation ratio of doublet excitons was up to 70%. This study provides a new approach for designing high-performance luminescence biphenylmethyl radicals for applications in organic electroluminescence.


 Please wait while we load your content...
Please wait while we load your content...