Quasimolecular complexes in the CuxTiSe2−ySy intercalation compound
Abstract
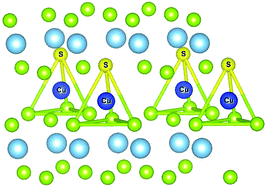
TiSe2 is a member of the layered transition metal dichalcogenides and displays a transition into the charge density wave (CDW) state upon cooling. The intercalation of copper into TiSe2 leads to a superconducting state transition. However, these electronic states are suppressed when both Cu and S are introduced into the TiSe2 crystal structure. The electronic structures of the copper intercalated CuxTiSe2−ySy (0 < y < 0.3) compounds have been studied using XPS, XAS and ResPES spectroscopic techniques and theoretical calculations. We show evidence suggesting that the copper atoms occupy sulfur coordinated tetrahedral sites in the interlayer space. This is accompanied by the formation of the Cu–S chemical bonds involving Ti 3d-orbitals and S 3d-orbitals. The suppression mechanism of the CDW and superconducting states in CuxTiSe2−ySy due to the sulfur for selenium substitution has been proposed considering that sulfur acts as a trap for copper atoms and does not allow Cu–TiSe2 bond formation.


 Please wait while we load your content...
Please wait while we load your content...