Configuration-dependent optical properties and acid susceptibility of azulene compounds†
Abstract
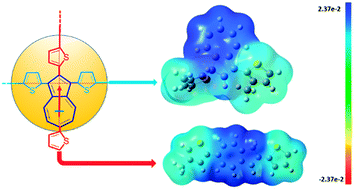
We report the unique optical and protonation characteristics of azulene compounds with different molecular configurations and demonstrate their potential application as acid sensor materials. The unique characteristic of azulene compounds is its large dipole moment. Azulene derivatives with conjugation either along or orthogonal to the dipole direction of azulene were synthesized, and their electronic and optical properties were studied. Our results show that azulene with conjugation orthogonal to the dipole direction exhibits significant change in optical properties upon protonation due to strong intermolecular charge transfer. The low band gap absorption can reach up to mid-IR range, albeit at high acid doping concentration. On the other hand, conjugated azulenes along the dipole direction could be easily protonated even at very low acid concentration (ppm level), which is attributed to the formation of co-planar structures upon protonation and to their high proton affinity. Mechanisms behind the discrepancy between the two configurations are elucidated and further supported by computer simulation. The application of azulene chromophores as a chemical sensor with sensitivity at ppm level was also demonstrated in this work.


 Please wait while we load your content...
Please wait while we load your content...