The contribution of hydrogen bonding to the photomechanical response of azobenzene-functionalized polyamides†
Abstract
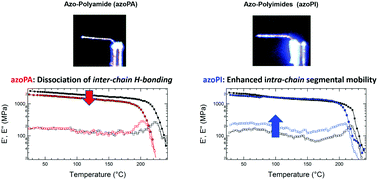
Photomechanical effects in materials can directly convert light stimulus into mechanical work. The magnitude of the photogenerated work is directly associated with the material stiffness (modulus). Here, we report the synthesis of an azobenzene-functionalized polyamide (azoPA) and demonstrate its room-temperature photomechanical response. The comparatively large photomechanical response of this high stiffness material is enabled by light-induced, azobenzene-assisted dissociation of inter-chain hydrogen bonding within the polymer backbone. The photomechanical response of the azoPA is directly contrasted with an analogous azobenzene-functionalized polyimide (azoPI) to illustrate the influence of intermolecular interactions.


 Please wait while we load your content...
Please wait while we load your content...