Mechanochemical nanoparticle functionalization for liquid crystal nanocomposites based on COOH-pyridine heterosynthons†
Abstract
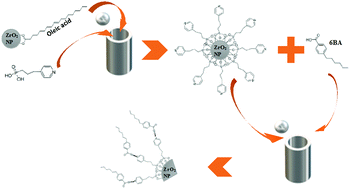
Nanoparticle/liquid crystal (NP/LC) composites can be stabilized by hydrogen bonding, a relatively strong, yet reversible interaction allowing for the annealing of defects. Previously, nanocomposites based on 4-hexylbenzoic acid (6BA) and ZrO2 NPs with pendant carboxylic acid groups were investigated, leading to problems of particle aggregation due to intra- and inter-particle hydrogen bonding. Here we report the synthesis of NP–LC composites based on different hydrogen bond acceptor and donor groups, promoting NP–LC coupling and reducing aggregation due to NP–NP interactions. Specifically, we developed a mechanochemical, solvent-free approach for efficient functionalization of ZrO2 NPs with (3-(pyridin-4-yl)propyl)phosphonic acid (3-PPA), leading to NPs with pendant pyridine groups that act as hydrogen bond acceptors only. This is the first example of using hydrogen bonded heterosynthons to tune the LC–NP interactions. The miscibility of the pyridine-functionalized NPs with 4-hexylbenzoic acid (6BA), a strong hydrogen bond donor, versus trans-4-butylcyclohexanecarboxylic acid (4-BCHA), a weaker hydrogen bond donor, was characterized by polarized optical and fluorescence microscopies. The specificity of the NP/LC acceptor/donor hydrogen bonds improves the miscibility over the NP/LC dispersions based on COOH dimer interactions only. The different effect of the NPs on the 4-BCHA properties as compared to 6BA can be related to the relative strengths of the COOH-pyridine hydrogen bonds.


 Please wait while we load your content...
Please wait while we load your content...