Control of oxygen vacancies in ZnO nanorods by annealing and their influence on ZnO/PEDOT:PSS diode behaviour†
Abstract
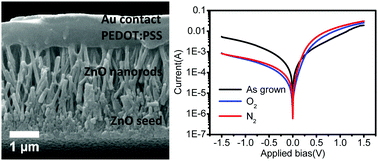
ZnO is one of the most widely studied semiconductors due to its direct wide band gap and high exciton binding energy. Due to its ease of synthesis, robustness and low cost, ZnO has been applied in a wide range of devices, including nanogenerators, solar cells, and photodetectors. In this work, ZnO nanorods were synthesized in a single step using an aqueous method at temperatures below 100 °C. The nanorods were annealed in oxygen and nitrogen and a p-type polymer poly(3,4-ethylenedioxythiophene)polystyrene sulfonate (PEDOT:PSS) was spray coated onto the top of ZnO nanorods to form a p–n junction. The I–V characteristics of the device showed that the annealing atmosphere had a significant effect on the rectification ratio of the device. Further analysis using Mott–Schottky, photoluminescence, and X-ray photoelectron spectroscopy (XPS) indicated that oxygen vacancy concentration correlated well with the free electron density in ZnO as well as the rectification ratio of the p–n junction devices. Devices made with ZnO nanorods annealed in nitrogen had a better rectification ratio than oxygen, representing a simple method to improve p–n junction diode behaviour through tuning the defect properties of the nanorods via controlled annealing.


 Please wait while we load your content...
Please wait while we load your content...