Eu(iii)-coordination polymer sub-micron fibers: material for selective and sensitive detection of Cu2+ ions via competition between photoinduced electron transfer and energy transfer†
Abstract
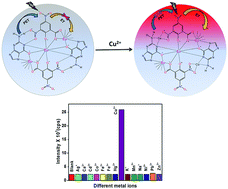
In this article, we report a unique strategy based on the competition between photoinduced electron transfer (PET) and intramolecular energy transfer (ET) processes occurring among the components of guanine-based europium(III) coordination polymer sub-micron fibers (CPMFs) for the selective and sensitive detection of Cu2+. The novel CPMFs were synthesized via a hydrothermal method using guanine, europium ions (Eu3+) and 3,5-dinitrosalicylic acid (DNSA) as an auxiliary linking molecule that can sensitize the luminescence of Eu3+ ions via intramolecular ET. The photoluminescence (PL) emission signal from the CPMFs is found to be weak due to the existence of PET from guanine to DNSA. The PET process from guanine to DNSA prevents the intramolecular ET from DNSA to Eu3+ ions, leading to the quenching of the luminescence from the CPMFs. However, 11 times enhancement of the PL emission signal from the CPMFs is observed in the presence of Cu2+ ions (12 μM) which is attributed to the suppression of the PET process (or enhancement of ET) as a result of coordination of Cu2+ with guanine molecules. This forms the basis of Cu2+ ion detection and the prepared CPMFs exhibited excellent selectivity and good sensitivity toward Cu2+ ions up to the 1.42 μM detection limit. The study was also extended to real tap water samples, where spiking with Cu2+ ions led to a 10 times enhancement of the PL emission intensity from the CPMFs, though hardly any change was noted for the unspiked tap water sample. We envision that our CPMFs are potential candidates for application in ultrasensitive time-resolved fluorometric assays owing to their long luminescence lifetime, good dispersion and stability in solution.


 Please wait while we load your content...
Please wait while we load your content...