Enzymatic synthesis of a thiolated chitosan-based wound dressing crosslinked with chicoric acid
Abstract
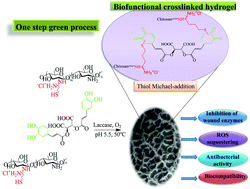
This work describes the enzymatic synthesis of multifunctional hydrogels for chronic wound treatment using thiolated chitosan and the natural polyphenol chicoric acid. Gelation was achieved by laccase-catalyzed oxidation of chicoric acid, a natural compound used for the first time as a homobifunctional crosslinker, reacting subsequently with nucleophilic thiol and amino groups from the chitosan derivative. This approach allowed for twice as fast gelation at a three-fold reduced crosslinking reagent concentration, compared to reported enzymatic synthesis of hydrogels using gallic acid as a phenolic provider. Hydrogels with 600% swelling capacity, coupled with only 20% weight loss after 6 days under physiological conditions, were obtained. The clinically relevant Gram-positive Staphylococcus aureus and the Gram-negative Pseudomonas aeruginosa were reduced by up to 4.5 and 5.5 logs, respectively. A tunable, in the range of 20–95%, ex vivo inhibition of myeloperoxidase (MPO) activity in chronic wound exudate was achieved, together with control over the total matrix metalloproteinase (MMP) activities.


 Please wait while we load your content...
Please wait while we load your content...