Hydrophobized SN38 to redox-hypersensitive nanorods for cancer therapy
Abstract
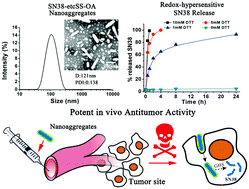
The clinical application of SN38 (7-ethyl-10-hydroxy-camptothecin) is severely restricted by its extremely low water solubility. Nanoaggregates formed by amphiphilic SN38 prodrugs have been widely used for the delivery of SN38. In this study, we used a hydrophobized SN38 prodrug, rather than a typical SN38 amphiphile, to construct rod-shaped nanoaggregates for efficient SN38 delivery. The hydrophobized SN38 was synthesized by conjugating SN38 with oleic acid using disulfanyl-ethyl carbonate as the linker. Interestingly, the resulting prodrug self-assembled into nanorods with high drug loading capacity (45%) and colloidal stability. Moreover, these nanorods displayed an impressively high redox-sensitivity to release 100% SN38 within 1 h in 10 mM DTT, versus 1% in phosphate buffer (pH 7.4). The efficient drug release resulted in an uncompromised in vitro cytotoxicity, which was comparable to free SN38 and nearly 93-fold more potent than CPT-11. Most importantly, these novel prodrug nanoaggregates exhibited potent antitumor activity in the CT26 colorectal cancer xenograft. The nanoaggregates of such redox-hypersensitive hydrophobized SN38 represent an effective alternative strategy for developing novel SN38 nanomedicines.


 Please wait while we load your content...
Please wait while we load your content...