Synthesis of a BODIPY disulfonate near-infrared fluorescence-enhanced probe with high selectivity to endogenous glutathione and two-photon fluorescent turn-on through thiol-induced SNAr substitution†
Abstract
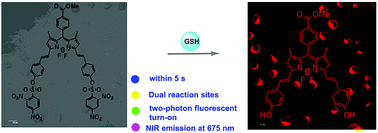
A BODIPY disulfonate BODIPY-diONs with two-photon fluorescent turn-on effect was developed as fluorescence probe for selective detection of glutathione over cysteine and homocysteine. BODIPY-diONs is weakly fluorescent due to the 2,4-dinitrobenzenesulfonyl quencher group. When GSH was added, a SNAr substitution reaction was triggered. The red emission of the BODIPY fluorophore at 675 nm was switched on, with a 27-fold emission enhancement in fluorescence intensity. The color of the solution changed from blue to green together with fluorescence appeared within 5 s. The absorbance and emission maxima of the probe BODIPY-diONs were achieved at 650 nm and 675 nm, respectively (quantum yield: 0.11). Interestingly, under the sapphire pulsed laser's 800 nm irradiation, in presence of GSH, the two-photon excited fluorescence (TPEF) of probe BODIPY-diONs was turned on, affording an OFF–ON response signal and a strong emission band at 682 nm. Furthermore, for detection of GSH, the chemodosimeter BODIPY-diONs exhibits high sensitivity and excellent anti-interference with low detection limit of 0.17 μM, and it works effectively within a wide pH range. Furthermore, the imaging studies proved that the probe BODIPY-diONs is suitable for the detection of GSH in complete physiological media.


 Please wait while we load your content...
Please wait while we load your content...