Main-chain polyacetal conjugates with HIF-1 inhibitors: temperature-responsive, pH-degradable drug delivery vehicles†
Abstract
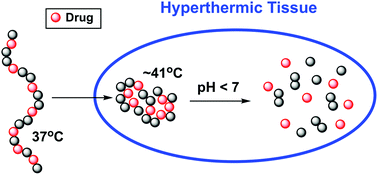
Main-chain polymer–drug conjugates are prepared from polyacetals (PA) and three hydrophobic diol-based HIF-1 inhibitors. The new conjugates are temperature-responsive with lower critical solution temperature (LCST) behavior and are intrinsically pH-degradable. While soluble in plasma at room temperature, they lose solubility above a target temperature that can be adjusted to virtually any temperature of physicological interest, providing mechanisms for site-specific delivery by active thermal targeting or temperature-induced gelation. The reverse phase transition temperature can be precisely tuned by proper choice of four structural variables that characterize the amphiphilic diol and divinyl ether monomers used in the synthesis, or by adjusting the content of drug incorporated within the polymer. These main-chain PA–drug conjugates also allow for site-specific controlled release as they degrade in acidic microenvironments such as tumors. The degradation rates increase with decreasing pH, degradation products are neutral, and pristine drug is released, without any remnants of the conjugation chemistry.


 Please wait while we load your content...
Please wait while we load your content...