A facile synthesis tool of nanoporous carbon for promising H2, CO2, and CH4 sorption capacity and selective gas separation†
Abstract
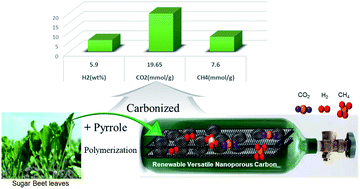
The commercialization of hydrogen as a clean source of energy is a vital requirement for overcoming the anticipated energy crisis. In addition, the capture of CO2 and commercialization of methane as an efficient and clean alternative to polluting gasoline are important goals. To this end, we have developed a nanoporous activated carbon material prepared from renewable resources that has a high storage capacity for various gases. Sugar beet leaves were converted to graphite flakes and decorated with polymer nanoparticles, giving rise to a highly porous activated carbon through chemical activation. The developed porous carbon has a high surface area (2800 m2 g−1) and specific pore volume (1.86 cm3 g−1), as well as high nitrogen and oxygen contents. The combination of high surface area, pore volume, and nitrogen and oxygen contents provided superior storage capacity for various gases. The total hydrogen storage capacities at 20 bar were 5.9 and 0.15 wt% at 77 and 298 K, respectively. In addition, the physical upper limit of hydrogen storage capacity was also evaluated using Brunauer–Emmett–Teller isotherms at the liquefaction temperature of hydrogen (20 K). A value of 14.1 wt% was obtained, which is the highest reported value for a porous carbon. The CO2 capture and CH4 storage capacities at room temperature and 20 bar were 19.65 and 7.6 mmol g−1, respectively, which are also among the highest values reported for porous carbon materials. Furthermore, the separation selectivity for CO2/CH4 binary mixtures was evaluated based on the ideal adsorbed solution theory (IAST) model and found to be 4.6.


 Please wait while we load your content...
Please wait while we load your content...