Water oxidation catalysed by quantum-sized BiVO4†
Abstract
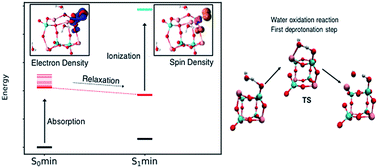
Bismuth vanadate BiVO4 is one of the most promising materials for photoelectrochemical water splitting, with recent work highlighting the improved photocatalytic activity of quantum sized BiVO4 compared with the crystalline phase. Herein, we report a theoretical investigation of the structural, optical and catalytic properties of the (BiVO4)4 clusters through a combination of density functional theory methods (ab initio molecular dynamics, time-dependent density functional theory, transition state theory). The enhanced solar water oxidation efficiency of BiVO4 quantum-sized clusters is linked with the localisation of the spin density on the cluster surface, and the dramatic reduction, compared with the crystalline BiVO4 phase, of the Gibbs energy of activation and Gibbs energy of reaction associated with the hydrogen transfer process between water and BiVO4. Our results illustrate the main effects associated with the reduction of dimensions (from bulk to quantum-size) on the main steps of water oxidation mechanisms. This understanding can contribute to the design of efficient BiVO4 quantum sized water-splitting photocatalysts.


 Please wait while we load your content...
Please wait while we load your content...