Hollow cobalt phosphide octahedral pre-catalysts with exceptionally high intrinsic catalytic activity for electro-oxidation of water and methanol†
Abstract
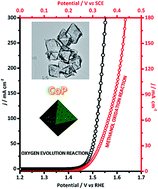
Microstructural engineering is an effective approach to improving electrocatalytic activity of a catalyst. Shape-controlled hollow nanostructures represent a class of interesting architectures for use in electrocatalysis, given that they may offer large surface area, preferably exposed active sites, reduced diffusion pathways for both charge and mass transport, as well as enhanced catalytic activity due to the nano-cavity effect. Herein, we for the first time report the synthesis of hollow cobalt phosphide nanoparticles with a well-defined octahedral shape (CoP OCHs) via multi-step reactions. When used as pre-catalysts for the oxygen evolution reaction (OER) in an alkaline solution, the as-obtained CoP OCHs not only show high apparent catalytic activity requiring an overpotential of only 240 mV to deliver the benchmark current density of 10 mA cm−2, but also exhibit an exceptionally high catalyst surface-area-based turnover frequency (TOF) of 17.6 s−1 and a catalyst mass-based TOF of 0.072 s−1 at a low overpotential of 300 mV, demonstrating excellent intrinsic catalytic activity. Moreover, the CoP OCHs also show high electrocatalytic activity for the methanol oxidation reaction (MOR), outperforming most metal phosphide based MOR catalysts reported so far. The synthetic strategy reported here can be readily extended to prepare other hollow shape-controlled metal phosphide catalysts.


 Please wait while we load your content...
Please wait while we load your content...