Silicon monophosphide as a possible lithium battery anode material†
Abstract
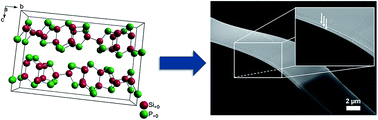
Binary (semi)metal–phosphorus compounds fascinate nowadays as they promise high specific capacities in metal ion batteries and an advantageous in situ formation of the electrochemically active material inside the electrochemical cell without air exposure. Here, we report on SiP as a new member of this material family which was tested as an anode material in Li ion batteries due to a highly attractive theoretical specific capacity of about 3000 mAh g−1 SiP. After synthesis by a vapour-transport reaction a cotton wool-like product was obtained revealing a layered 2D crystalline microribbon-like morphology, space group Cmc21, which may allow fast Li ion intercalation/diffusion kinetics. SiP also opens up a novel perspective for application in ultra-thin optoelectronics or flexible photovoltaics. During the first discharging half-cycle the crystalline phase amorphizes as indicated by operando synchrotron powder diffraction followed by an amorphous state without re-formation of any crystalline phase, including Li3P. Our investigations after 50 discharging–charging cycles show a specific capacity of 550 mAh g−1, which supports the trend of lifetime reduction by LiP formation. Further optimisation is necessary to allow a broad application for novel technologies like high performance Li ion or Li–S batteries.


 Please wait while we load your content...
Please wait while we load your content...