A comparative study of metal (Ni, Co, or Mn)-borate catalysts and their photodeposition on rGO/ZnO nanoarrays for photoelectrochemical water splitting†
Abstract
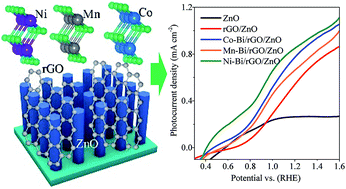
Feasible and efficient photoelectrochemical (PEC) water splitting demands a rational integration of solar light absorbers with active electrocatalysts. Herein, we first compare three amorphous metal-borates (M-Bi, M = Ni, Co, Mn) as low-cost electrocatalysts, among which Mn-Bi is proposed for the first time for fabrication of new PEC devices for oxygen evolution reaction (OER). Density functional theory (DFT) calculations compared the catalytic activity of the effective structures in M-Bi and found that NiO6 possesses kinetically the lowest overall OER energy barrier. Experimentally, M-Bi thin layers were self-assembled on reduced graphene oxide (rGO) linked ZnO nanorod arrays respectively, to form a highly efficient ternary PEC system (M-Bi/rGO/ZnO) using a modified photodeposition method. rGO facilitates the fast charge separation in light-absorbing ZnO NAs, while M-Bi (M = Ni, Co, Mn) can improve the kinetics of OER. In accordance with DFT results, Ni-Bi serves as the most active electrocatalyst in such a PEC device, followed by Co-Bi and Mn-Bi. Compared to ZnO, the photoelectroconversion efficiency is elevated by approximately 4 times on Ni-Bi/rGO/ZnO, with its onset potential migrated by 0.17 V in the cathodic direction under one sun illumination.


 Please wait while we load your content...
Please wait while we load your content...