Thermally driven in situ exsolution of Ni nanoparticles from (Ni, Gd)CeO2 for high-performance solid oxide fuel cells†
Abstract
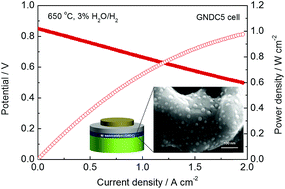
In situ exsolution of metal nanoparticles affords a high content and uniform distribution of metal nanocatalysts without complex synthetic processes. To implement this strategy in practical electrodes for solid oxide fuel cells, understanding the exsolution process in terms of synthesis temperature and atmosphere is a prerequisite. Herein, we demonstrate that Ni:Gd co-doped ceria (GNDC) can be effectively used as an in situ exsolution system, from which substitutionally doped Ni is thermally exsolved as NiO nanoparticles strongly attached to the surface of GNDC, the host oxide, and subsequently reduced to a Ni nanocatalyst under anodic operation conditions. The exsolution procedures were characterized by X-ray diffraction, Raman spectroscopy, and transmission electron spectroscopy, which revealed that the evolution of Ni nanoparticles could be solely controlled by thermal treatment. The thermally exsolved Ni nanocatalyst from the 5 mol% Ni-doped GNDC electrode exhibits a polarization resistance comparable to that of the mechanically mixed Ni-GDC composite electrode, with a significant increase in the calculated triple phase boundary density despite having a low Ni volume fraction. By employing the GNDC layer as a functional layer of an anode-supported SOFC, we demonstrated the utilization of the thermally exsolved Ni nanocatalyst combined with fluorite-structured doped-ceria as an electrode of SOFCs at low temperature.


 Please wait while we load your content...
Please wait while we load your content...