Effect of halide ratio and Cs+ addition on the photochemical stability of lead halide perovskites†
Abstract
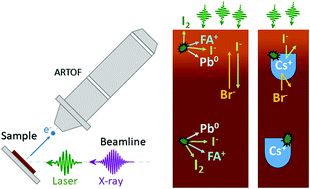
Lead halide perovskite solar cells with multi-cation/mixed halide materials now give power conversion efficiencies of more than 20%. The stability of these mixed materials has been significantly improved through the addition of Cs+ compared to the original methylammonium lead iodide. However, it remains one of the most significant challenges for commercialisation. In this study, we use photoelectron spectroscopy (PES) in combination with visible laser illumination to study the photo-stability of perovskite films with different compositions. These include Br : I ratios of 50 : 50 and 17 : 83 and compositions with and without Cs+. For the samples without Cs and the 50 : 50 samples, we found that the surface was enriched in Br and depleted in I during illumination and that some of the perovskite decomposed into Pb0, organic halide salts, and iodine. After illumination, both of these reactions were partially reversible. Furthermore, the surfaces of the films were enriched in organic halide salts indicating that the cations were not degraded into volatile products. With the addition of Cs+ to the samples, photo-induced changes were significantly suppressed for a 50 : 50 bromide to iodide ratio and completely suppressed for perovskites with a 17 : 83 ratio at light intensities exceeding 1 sun equivalent.
- This article is part of the themed collection: Journal of Materials Chemistry A Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...