Dense thiol arrays for metal–organic frameworks: boiling water stability, Hg removal beyond 2 ppb and facile crosslinking†
Abstract
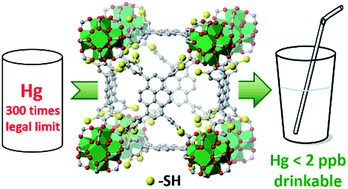
We report a breakthrough in the synthesis of metal–organic frameworks (MOFs) extensively equipped with thiol groups. The main motivation arises from the versatile reactivity of the thiol function, which imparts to the porous host unique potential in metal-uptake, catalytic and electronic properties. The synthesis of thiol-equipped molecules for MOF construction, however, remains challenging, because of the lack of efficient, wide-scope synthetic protocols. By utilizing nucleophilic substitution between benzyl thiol and aromatic halides, and AlCl3-promoted deprotection of the resultant benzyl thioether, we succeeded in attaching thiol groups onto the carboxylate linker backbone in a dense array. The widened access of thiol-equipped MOFs facilitates in-depth exploration of open framework sulfur chemistry, achieving, for instance, fast removal of Hg to below the drinking limit of 2 ppb.


 Please wait while we load your content...
Please wait while we load your content...