New challenges of electrokinetic studies in investigating the reaction mechanism of electrochemical CO2 reduction†
Abstract
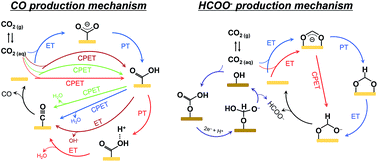
An electrokinetic analysis of electrochemical CO2 reduction reactions provides information about the decoupled involvement of electron–proton transfer from the Tafel slope and reaction order analyses. Conventionally, a one-electron transfer to CO2 and a chemical proton transfer from HCO3− have been considered to be typical rate-limiting steps. These suggested reaction mechanisms are justified under several assumptions: (1) the bicarbonate ion is a major proton donor, (2) the gaseous CO2 is a carbon source, (3) the reaction mechanism is unaffected by the applied potentials outside the Tafel region, etc. However, recent electrokinetic studies combined with in situ and isotopic experiments raise a question that the above conventional assumptions may not always be valid. Furthermore, there are unresolved issues between the mechanisms suggested by electrokinetic studies. In this review, reported reaction mechanisms of the CO2 reduction reaction are summarized with CO and HCOO− formation as model reaction systems. The reaction pathways are also discussed with a theoretical consideration. A deep investigation into the mechanisms reveals the complex feature of reaction pathways and the difficulty in suggesting the mechanism solely from an electrokinetic analysis.
- This article is part of the themed collection: Recent Review Articles


 Please wait while we load your content...
Please wait while we load your content...