Superionically conducting β′′-Al2O3 thin films processed using flame synthesized nanopowders†
Abstract
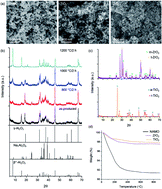
Commercial β′′-Al2O3 solid electrolytes for Na+ batteries are exclusively produced in tubular form, in part, due to processing difficulties. High sintering temperatures combined with Na2O loss and excessive grain growth complicate processing of β′′-Al2O3 thin films (<100 μm) which could potentially reduce cell resistance, increase energy density, and even permit room temperature operations. In this study, we use high surface area flame made nanopowders at selected compositions to drive densification to produce β′′-Al2O3 thin films with controlled microstructures and Na2O loss to maintain a high β′′-Al2O3 fraction. We show that addition of TiO2 and ZrO2 dramatically enhances the sintering kinetics, resulting in dense (>95%), thin (≤50 μm) β′′-Al2O3 films at the lowest sintering temperature ever reported (1320 °C/2 h). The sintered films also offer superionic conductivity (>1 mS cm−1) at room temperature. Symmetrical cell (Na/β′′-Al2O3/Na) testing further comfirms its potential utility in Na batteries using β′′-Al2O3 solid electrolytes.


 Please wait while we load your content...
Please wait while we load your content...