High oxide-ion conductivity in Si-deficient La9.565(Si5.826□0.174)O26 apatite without interstitial oxygens due to the overbonded channel oxygens†
Abstract
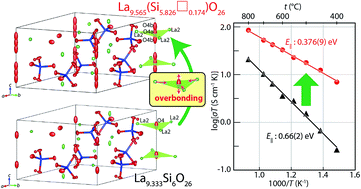
Apatite-type rare-earth silicates are attractive materials with extensive applications such as in solid-oxide fuel cells due to their extremely high oxide-ion conductivity below 600 °C. The presence of interstitial (excess) oxygens has been believed to be responsible for the high conductivity of apatite-type materials. On the contrary, the present study clearly reveals the presence of Si vacancies □ instead of interstitial oxygens in La-rich La9.565(Si5.826□0.174)O26 using single-crystal neutron and X-ray diffraction analyses, density measurements and ab initio electronic calculations. Higher mobility (i.e., lower activation energy) of oxide ions along the c axis is a major reason for the high oxide-ion conductivity of La9.565(Si5.826□0.174)O26 when compared with that of La9.333Si6O26. Excess La cations yield overbonded channel oxygens, leading to their highly anisotropic atomic displacements and high oxygen mobility along the c axis. This novel finding of the overbonding effect without interstitial oxygens will open a new window for the design of better ion conductors.


 Please wait while we load your content...
Please wait while we load your content...