Doped ordered mesoporous carbons as novel, selective electrocatalysts for the reduction of nitrobenzene to aniline†
Abstract
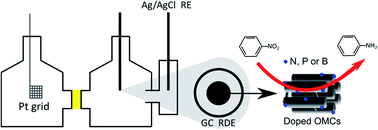
Ordered mesoporous carbons (OMCs) doped with nitrogen, phosphorus or boron were synthesised through a two-step nanocasting method and studied as electrocatalysts for the reduction of nitrobenzene to aniline in a half-cell setup. The nature of the dopant played a crucial role in the electrocatalytic performance of the doped OMCs, which was monitored by LSV with a rotating disk electrode setup. The incorporation of boron generated the electrocatalysts with the highest kinetic current density, whereas the incorporation of phosphorus led to the lowest overpotential. Doping with nitrogen led to intermediate behaviour in terms of onset potential and kinetic current density, but provided the highest selectivity towards aniline, thus resulting in the most promising electrocatalyst developed in this study. Density functional theory calculations allowed explaining the observed difference in the onset potentials between the various doped OMCs, and indicated that both graphitic N and pyrdinic N can generate active sites in the N-doped electrocatalyst. A chronoamperometric experiment over N-doped OMC performed at −0.75 V vs. Fc/Fc+ in an acidic environment, resulted in a conversion of 61% with an overall selectivity of 87% to aniline. These are the highest activity and selectivity ever reported for an electrocatalyst for the reduction of nitrobenzene to aniline, making N-doped OMC a promising candidate for the electrochemical cogeneration of this industrially relevant product and electricity in a fuel cell setup.


 Please wait while we load your content...
Please wait while we load your content...