Physicochemical properties of nanostructured Pd/lanthanide-doped ceria spheres with high catalytic activity for CH4 combustion†
Abstract
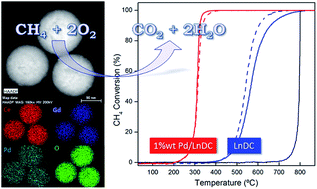
In this work, nanostructured Ce0.9Gd0.1O2−δ (GDC) and Ce0.9Pr0.1O2−δ (PrDC) spheres previously obtained by microwave assisted hydrothermal homogeneous co-precipitation were impregnated with 1 wt% Pd by the incipient wetness impregnation by an aqueous Pd2+ solution. Their properties were characterized by synchrotron radiation X-ray diffraction (SR-XRD), X-ray absorption near-edge spectroscopy (XANES) and scanning and high resolution electron microscopy with X-ray spectroscopy. Spherical particles with average diameters around 200 nm were found to consist of crystallites of average size, 10 nm, with small particles of PdO finely dispersed over the sphere surface. In situ XRD and XANES experiments were carried out under reducing and oxidizing conditions in order to investigate the redox behaviour of these materials and to evaluate the effect of Pd loading on the oxidation state of Ce. All of the lanthanide-doped ceria (LnDC) supports were found to have a cubic crystal structure (Fm3m space group). An increase in lattice parameters was observed under reducing conditions which was attributed to the reduction of Ce4+ ions to the larger Ce3+ ions, and to the associated increase in oxygen vacancy (VO) concentration. Addition of Pd to the LnDC spheres increased their Ce3+ content. Finally, catalytic tests for CH4 combustion were performed on the LnDC and Pd/LnDC nanocatalysts. The best performance was observed in samples with 1 wt% Pd loading, which exhibited T50 values (temperature at which 50% of CH4 conversion was reached) close to 310 °C. These values are 220 °C and 260 °C lower than those obtained for nanostructured PrDC and GDC spheres alone, respectively.


 Please wait while we load your content...
Please wait while we load your content...