Anti-perovskite cathodes for lithium batteries†
Abstract
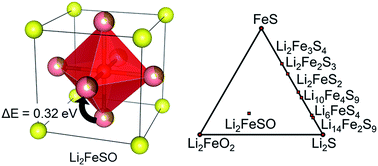
It was recently discovered that Li2FeChO (Ch = S, Se, Te) anti-perovskites exhibit an outstanding rate capability and a good discharge capacity as Li-ion battery cathodes. In this work, we use density functional theory calculations to study the origin of the electrochemical characteristics of anti-perovskite cathodes using Li2FeSO as a model material. We evaluate the phase stability, ion-transport, and structural stability of this material and, interestingly, found that Li2FeSO is meta-stable at 0 K. The experimentally observed anti-perovskite phase likely originates from either entropy stabilization or sluggish decomposition kinetics. When delithiated, the stability of the material worsens. Additionally, over-delithiation may lead to irreversible structural change. The good rate capability of Li2FeSO is attributed to the high Li conductivity, where the low Li ion diffusion barrier (∼0.32 eV) rivals those of state-of-art superionic Li conductors. In the search for materials with a voltage higher than that of Li2FeSO, we screen Co, Cr, Cu, Mn, Mo, Ni, and V substitutions into the Fe site of Li2FeSO. While Cu and Ni substitutions may offer higher average voltages, the corresponding materials are less stable than Li2FeSO.


 Please wait while we load your content...
Please wait while we load your content...