Tuning the structural stability of LiBH4 through boron-based compounds towards superior dehydrogenation†
Abstract
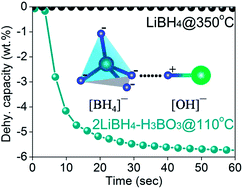
The remarkable destabilization effects of H3BO3, HBO2, and B2O3 on dehydrogenation of LiBH4 are revealed in this work. The effectiveness of destabilizing the structural stability is in the order of H3BO3 > HBO2 > B2O3. Besides, through optimizing the molar ratio of LiBH4 and H3BO3 and milling treatment, the destabilization effect, especially for dehydrogenation kinetics, is further enhanced. For example, at a temperature as low as 110 °C, 5.8 wt% hydrogen can be liberated in seconds from 2LiBH4–H3BO3 prepared through pre-milling. The investigation reveals that each of the LiBH4–H3BO3, LiBH4–HBO2 and LiBH4–B2O3 systems undergo multiple dehydrogenation stages corresponding to different destabilization mechanisms. The reaction at lower temperature is ascribed to the H+⋯H− coupling mechanism which should be enhanced by the [OH]−⋯[BH4]− interaction mode. Pre-milling treatment of LiBH4 and H3BO3 also promotes the H+⋯H− interaction which may have originated from the increasing contact area as a result of the fine particles, and therefore probably reduced the reaction activation energy. Consequently, it gives rise to the superior dehydrogenation performance of lower temperature, rapid kinetics, pure hydrogen and high capacity, which are required for off-board hydrogen energy vehicle application.


 Please wait while we load your content...
Please wait while we load your content...