MOF-based ternary nanocomposites for better CO2 photoreduction: roles of heterojunctions and coordinatively unsaturated metal sites†
Abstract
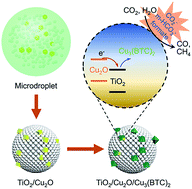
Semiconductors are the most widely used catalysts for CO2 photoreduction. However, their efficiencies are limited by low charge carrier density and poor CO2 activation. Towards solving these issues, a metal–organic framework (MOF)-based ternary nanocomposite was synthesized through self-assembly of TiO2/Cu2O heterojunctions via a microdroplet-based approach followed by in situ growth of Cu3(BTC)2 (BTC = 1,3,5-benzenetricarboxylate). With increased charge carrier density and efficient CO2 activation, the hybrid ternary nanocomposite exhibits a high CO2 conversion efficiency and preferential formation of CH4. Systematic measurements by using gas chromatography, photoluminescence spectroscopy, X-ray photoelectron spectroscopy, and time-resolved in situ diffuse reflectance infrared Fourier transform spectroscopy reveal that the semiconductor heterojunction and the coordinatively unsaturated copper sites within the hybrid nanostructure are attributable to the performance enhancements.


 Please wait while we load your content...
Please wait while we load your content...