Rhombohedral NASICON-type NaxFe2(SO4)3 for sodium ion batteries: comparison with phosphate and alluaudite phases†
Abstract
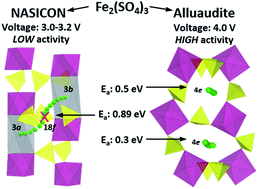
Sulfate has attracted considerable interest as an anion for cathode materials in lithium and sodium ion batteries because they induce high voltages. Herein, Fe2(SO4)3 in the rhombohedral NASICON phase was examined as a Na-ion cathode. Electrochemical, structural, and theoretical methods were employed. Starting from the charged state, Fe2(SO4)3 can be discharged to near theoretical capacity under very slow GITT experimental conditions. However, the available capacity rapidly dropped under high current. First-principles calculations found that one of the alkali metal sites was metastable and a high diffusion barrier of 0.9 eV for Na+ ion migration was found. The NASICON-type structure, with its open framework, is generally considered a good ionic conductor. However, the small size of SO42− anions limit the site availability and the mobility of Na+ ions in the NASICON phase of Fe2(SO4)3, which accounted for their inferior properties compared with the recently discovered alluaudite polymorph.


 Please wait while we load your content...
Please wait while we load your content...