Evaporation and morphological patterns of bi-dispersed colloidal droplets on hydrophilic and hydrophobic surfaces
Abstract
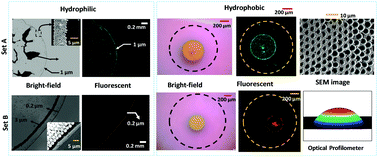
Understanding the formation of different morphological patterns depending on the particle size and surface wettability has great relevance in the separation, mixing and concentration of micro/nano particles and biological entities. We report the evaporation and morphological patterns of evaporating bi-dispersed colloidal droplets on hydrophilic and hydrophobic surfaces. To explain the underlying mechanisms of various particle distribution patterns, we propose a phenomenological model that accounts for the drag force, van der Waals and electrostatic interaction forces, and surface tension force acting on the particles. In the case of the hydrophilic surface (θ ∼ 27°), there is a competition between the frictional force arising due to the van der Waals (∼10−8 N) and electrostatic interaction forces (∼10−10 N) and the surface tension force (∼10−7 N) that depends on the particle size. Consequently, the smaller particles (0.2 and 1.0 μm in diameter) are found to be pinned and form an outer ring at the contact line whereas the larger particles (3.0 and 6.0 μm in diameter) move inward, either forming an inner ring or flocculating depending on the particle size. Interestingly, a completely different morphological pattern of the micro/nano particles is observed on a hydrophobic substrate (θ ∼ 110°): contact line pinning is no longer observed and particles form a centralized deposition pattern. The order of the magnitude of the surface tension force is higher as compared to the frictional force (∼10−8 N); thus the particles are driven radially inward and accumulate at the center of the droplet. Owing to the mixed mode of evaporation toward the end of evaporation, only a fraction of smaller particles travel radially outward due to the coffee-ring effect. Scanning electron microscopy images reveal that smaller particles are present mostly at the center with a small fraction of smaller particles at the edge of the pattern, whereas larger particles are uniformly distributed throughout.


 Please wait while we load your content...
Please wait while we load your content...