Morphology modulation in evaporative drying mediated crystallization of sodium chloride solution droplet with surfactant†
Abstract
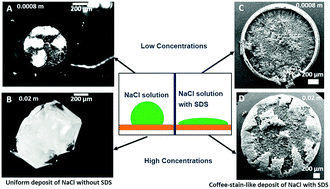
We report the evaporative drying of an aqueous droplet containing a dilute solution of sodium chloride (NaCl) on a hydrophobic substrate made of cross-linked poly-dimethyl siloxane (PDMS). The salt concentration Cn was varied between 0.08 molar (M) and 2.0 M. The contact line of the evaporating droplets shows significant initial retraction for all Cn, before they get pinned. While the final morphology comprises a few small NaCl crystals deposited around the pinned contact line, in droplets with a low Cn (<0.5 M), it transforms to a single large salt crystal when Cn > 0.7 M with no peripheral deposition. We further show that the deposition morphology drastically changes when an anionic surfactant, sodium dodecyl sulfate (SDS), is added into the salt-solutions. Even in the surfactant-laden droplets, the final deposition morphology changes significantly as a function of Cn. It transforms from a thick SDS ring surrounding a fractal-like deposit of NaCl crystallites at lower Cn to a peripheral deposit of NaCl crystals at higher Cn due to competition between micelle formation and crystallization. However, the crystallographic orientation of the deposited NaCl crystals remains unaltered irrespective of the presence of surfactant.


 Please wait while we load your content...
Please wait while we load your content...