Preparation and characterisation of highly alkaline hydrogels for the re-alkalisation of carbonated cementitious materials
Abstract
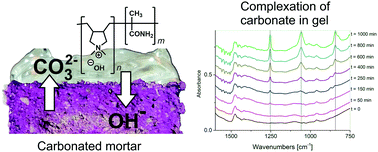
Highly alkaline hydrogels that allow the restoration of alkaline buffer in cementitious materials can be obtained from diallyldimethylammonium hydroxide. The latter must be prepared in dilute solutions and polymerised at ambient temperatures in order to avoid decomposition. Using methacrylamide as a neutral co-monomer capable of forming hydrogen bonds, the rheological properties of the gels can be adjusted over a wide range; e.g. the viscosity increases a thousandfold from 0.35 Pa s to >350 Pa s by using 10 mol% methacrylamide. For the proof of principle experiments, gels with 9 mol% methacrylamide were used, which contain approx. 1.6 mol hydroxide ions per kg gel. Ion exchange between this and a neutral chloride containing gel provided an apparent diffusion coefficient of 4.12 × 10−7 m2 s−1 for the hydroxide ions and confirmed the transport of chloride ions into the alkaline gel. The re-alkalisation was tested on fully carbonated mortar prisms made from Portland cement. Re-alkalisation of the mortar was confirmed by the phenolphthalein test according to DIN EN 14630:2007-01 and by a control experiment with pure calcium carbonate using IR spectroscopy.


 Please wait while we load your content...
Please wait while we load your content...