Synthetic hydrogels formed by thiol–ene crosslinking of vinyl sulfone-functional poly(methyl vinyl ether-alt-maleic acid) with α,ω-dithio-polyethyleneglycol†
Abstract
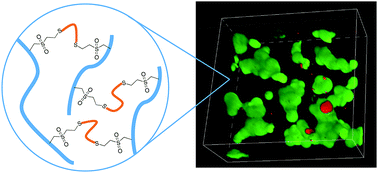
Polymer hydrogels formed by rapid thiol–ene coupling of macromolecular gel formers can offer access to versatile new matrices. This paper describes the efficient synthesis of cysteamine vinyl sulfone (CVS) trifluoroacetate, and its incorporation into poly(methyl vinyl ether-alt-maleic anhydride) (PMMAn) to form a series of CVS-functionalized poly(methyl vinyl ether-alt-maleic acid) polymers (PMM-CVSx) containing 10 to 30 mol% pendant vinyl sulfone groups. Aqueous mixtures of these PMM-CVS and a dithiol crosslinker, α,ω-dithio-polyethyleneglycol (HS-PEG-SH, Mn = 1 kDa), gelled through crosslinking by Michael addition within seconds to minutes, depending on pH, degree of functionalization, and polymer loading. Gelation efficiency, Young's modulus, equilibrium swelling and hydrolytic stability are described, and step-wise hydrogel post-functionalization with a small molecule thiol, cysteamine, was demonstrated. Cytocompatibility of these crosslinked hydrogels towards entrapped 3T3 fibroblasts was confirmed using a live/dead fluorescence assay.


 Please wait while we load your content...
Please wait while we load your content...