Ligand-regulated oligomerisation of allosterically interacting proteins†
Abstract
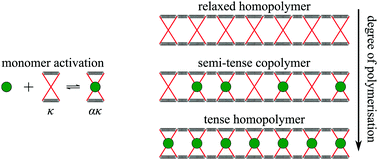
The binding of ligands to distinct sites at proteins or at protein clusters is often cooperative or anti-cooperative due to allosteric signalling between those sites. The allostery is usually attributed to a configurational change of the proteins from a relaxed to a configurationally different tense state. Alternatively, as originally proposed by Cooper and Dryden, a tense state may be achieved by merely restricting the thermal vibrations of the protein around its mean configuration. In this work, we provide theoretical tools to investigate fluctuation allostery using cooling and titration experiments in which ligands regulate dimerisation, or ring or chain formation. We discuss in detail how ligands may regulate the supramolecular (co)polymerisation of liganded and unliganded proteins.


 Please wait while we load your content...
Please wait while we load your content...