Ionic liquid mediated micelle to vesicle transition of a cationic gemini surfactant: a spectroscopic investigation†
Abstract
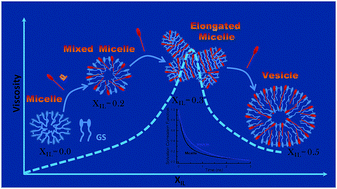
In this contribution, we have examined a composition dependent self aggregated structural modification of a catanionic mixture of the surface active ionic liquid (IL) 1-butyl-3-methylimidazolium octyl sulphate and a cationic gemini surfactant (14-5-14) in aqueous medium. We have observed that the hydrodynamic diameter of the aggregates increases with increasing IL concentration and microscopic evidence (HRTEM, FESEM, and LCSM) shows the formation of vesicle like aggregates (Dh ≈ 200 nm) at XIL = 0.5. The steady state fluorescence anisotropy of the membrane binding probe DPH shows a micelle to vesicle transition at this composition. The viscosity of the solution shows a peak at XIL = 0.3, indicating the formation of a worm like micelle as an intermediate of the micelle to vesicle transition. The rotational dynamics shows a stiffer surfactant packing in the vesicles compared to the micelles, whereas, the solvation dynamics measurements indicate a higher abundance of bound type water in the vascular medium compared to that for the micelle. The formed vesicles also show stability towards temperature and biomolecules, which can be used for respective applications.


 Please wait while we load your content...
Please wait while we load your content...