Efficient hydrogen generation from water using nanocomposite flakes based on graphene and magnesium†
Abstract
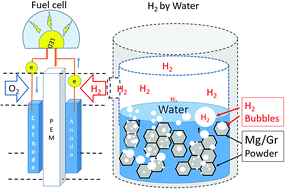
Water, through a metal–water reaction, is an appealing candidate to store and release hydrogen (H2), in particular as a portable, easy to use energy storage source. However, the release of hydrogen from the reaction of water with light metals can be violent or be inhibited by the formation of an oxide layer as is the case with magnesium (Mg). For this reason, we studied the use of graphene powder (Gr) as a nano-support to control the reactivity of Mg in water. Nanofilms and nanoparticles of Mg have been grown directly on graphene sheets inducing an increase of Mg surface area and the formation of metallic nanostructures that enhance the responsiveness of metal in water. We surprisingly observed that the water–Mg/Gr reaction happens at room temperature with impressive H2 production (the Mg/Gr hydrogen gravimetric density is 3%). Therefore, the hydrogen generated from water has been successfully used in a fuel cell proving that Mg/Gr is a promising material for on-demand H2 generation.


 Please wait while we load your content...
Please wait while we load your content...