Organic chemistry at anodes and photoanodes
Abstract
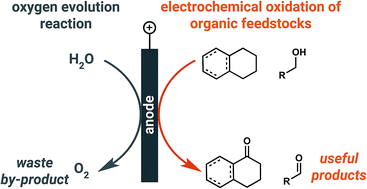
Solar-driven electrolytic water splitting is a promising means of storing renewable electricity, but the kinetic limitations of the anodic oxygen evolution reaction (OER) have impeded the deployment of electrolyzers that produce hydrogen fuels derived from water. In this review, we summarize alternative anodic chemistries being considered as a means of lowering the amount of electricity required to produce hydrogen at the cathode, or simply driving chemistry that forms products more valuable than oxygen at the anode. The potential for an organic oxidation reaction to instead occur at the anode presents a new opportunity for the production of value-added chemical products from cheap, readily available and, in some cases, renewable feedstocks.
- This article is part of the themed collection: Artificial Photosynthesis - From Sunlight to Fuels and Valuable Products for a Sustainable Future


 Please wait while we load your content...
Please wait while we load your content...