Solid-phase synthesis and electrochemical pseudo-capacitance of nitrogen-atom interstitial compound Co3N
Abstract
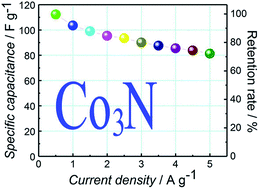
Metal nitrides have great potential for electrochemical energy storage, but are relatively scarcely investigated. Herein, a novel metal nitride, Co3N, is prepared by nitridation using a Co3O4 precursor heated at 500 °C for 2 h in flowing NH3, and characterized by using X-ray diffraction (XRD), field emission-scanning electron microscopy (FE-SEM), transmission electron microscopy (TEM), selected area electron diffraction (SAED) and energy dispersive spectroscopy (EDS) methods. Besides, the electrochemical properties of Co3N are investigated using cyclic voltammetry (CV), galvanostatic charge–discharge (GCD) and electrochemical impedance spectroscopy (EIS). The as-prepared Co3N demonstrates remarkable electrochemical performance with a high specific capacitance of 112.3 F g−1 at a current density of 0.5 A g−1, good rate capability (72.4%, from 0.5 to 5 A g−1) and superior cycling stability (109.7% retention over 10 000 cycles at a current density of 2 A g−1). Finally, a Co3N//activated carbon (AC) asymmetric supercapacitor (ASC) is successfully assembled by using Co3N and AC as the positive electrode and negative electrode, respectively. The as-fabricated ASC device can achieve a maximum energy density of 12.1 W h kg−1 at a power density of 204.6 W kg−1, which shows that the Co3N material should be a potential electrode material for supercapacitors.


 Please wait while we load your content...
Please wait while we load your content...