Biocatalyst–artificial metalloenzyme cascade based on alcohol dehydrogenase†
Abstract
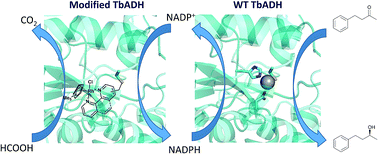
Chemo-enzymatic cascades of enzymes with transition metal catalysts can offer efficient synthetic strategies, but their development is challenging due to the incompatibility between proteins and transition metal complexes. Rhodium catalysts can be combined with alcohol dehydrogenases to regenerate nicotinamide cofactors using formate as the hydride donor. However, their use is limited, due to binding of the metals to residues on the enzyme surface, leading to mutual enzyme and catalyst inactivation. In this work, we replaced the zinc from Thermoanaerobacter brockii alcohol dehydrogenase (TbADH) with Rh(III) catalysts possessing nitrogen donor ligands, by covalent conjugation to the active site cysteine, to create artificial metalloenzymes for NADP+ reduction. TbADH was used as protein scaffold for both alcohol synthesis and the recycling of the cofactor, by combination of the chemically modified species with the non-modified recombinant enzyme. Stability studies revealed that the incorporation of the catalysts into the TbADH pocket provided a shielding environment for the metal catalyst, resulting in increased stability of both the recycling catalyst and the ADH. The reduction of a representative ketone using this novel alcohol dehydrogenase–artificial formate dehydrogenase cascade yielded better conversions than in the presence of free metal catalyst.
- This article is part of the themed collection: 2018 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...