Simultaneous nitrosylation and N-nitrosation of a Ni-thiolate model complex of Ni-containing SOD†
Abstract
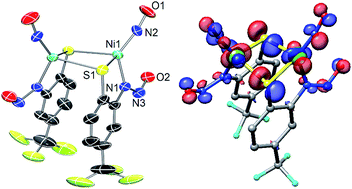
Nitric oxide (NO) is used as a substrate analogue/spectroscopic probe of metal sites that bind and activate oxygen and its derivatives. To assess the interaction of superoxide with the Ni center in Ni-containing superoxide dismutase (NiSOD), we studied the reaction of NO+ and NO with the model complex, Et4N[Ni(nmp)(SPh-o-NH2-p-CF3)] (1; nmp2− = dianion of N-(2-mercaptoethyl)picolinamide; −SPh-o-NH2-p-CF3 = 2-amino-4-(trifluoromethyl)benzenethiolate) and its oxidized analogue 1ox, respectively. The ultimate products of these reactions are the disulfide of −SPh-o-NH2-p-CF3 and the S,S-bridged tetrameric complex [Ni4(nmp)4], a result of S-based redox activity. However, introduction of NO to 1 affords the green dimeric {NiNO}10 complex (Et4N)2[{Ni(κ2-SPh-o-NNO-p-CF3)(NO)}2] (2) via NO-induced loss of nmp2− as the disulfide and N-nitrosation of the aromatic thiolate. Complex 2 was characterized by X-ray crystallography and several spectroscopies. These measurements are in-line with other tetrahedral complexes in the {NiNO}10 classification. In contrast to the established stability of this metal-nitrosyl class, the Ni–NO bond of 2 is labile and release of NO from this unit was quantified by trapping the NO with a CoII–porphyrin (70–80% yield). In the process, the Ni ends up coordinated by two o-nitrosaminobenzenethiolato ligands to result in the structurally characterized trans-(Et4N)2[Ni(SPh-o-NNO-p-CF3)2] (3), likely by a disproportionation mechanism. The isolation and characterization of 2 and 3 suggest that: (i) the strongly donating thiolates dominate the electronic structure of Ni-nitrosyls that result in less covalent Ni–NO bonds, and (ii) superoxide undergoes disproportionation via an outer-sphere mechanism in NiSOD as complexes in the {NiNO}9/8 state have yet to be isolated.


 Please wait while we load your content...
Please wait while we load your content...