Catalyst-dependent selectivity in sulfonium ylide cycloisomerization reactions†
Abstract
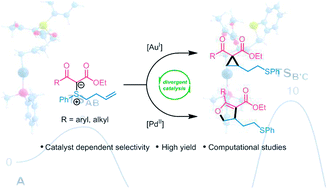
Divergent catalysis is an emerging field whereby access to structurally diverse compounds from a common precursor is achieved through controlled reaction pathways. Herein we present an unusual example of π-acid catalyst dependent selectivity in the cycloisomerization of alkene-tethered sulfonium ylides. Computational mechanistic studies revealed how the ability of palladium to cycle through oxidation states largely controls the selectivity.
- This article is part of the themed collection: New reactivity in organic chemistry


 Please wait while we load your content...
Please wait while we load your content...