Transition metal-free direct dehydrogenative arylation of activated C(sp3)–H bonds: synthetic ambit and DFT reactivity predictions†
Abstract
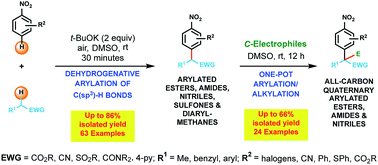
A transition metal-free dehydrogenative method for the direct mono-arylation of a wide range of activated C(sp3)–H bonds has been developed. This operationally simple and environmentally friendly aerobic arylation uses tert-BuOK as the base and nitroarenes as electrophiles to prepare up to gram quantities of structurally diverse sets (>60 examples) of α-arylated esters, amides, nitriles, sulfones and triaryl methanes. DFT calculations provided a predictive model, which states that substrates containing a C(sp3)–H bond with a sufficiently low pKa value should readily undergo arylation. The DFT prediction was confirmed through experimental testing of nearly a dozen substrates containing activated C(sp3)–H bonds. This arylation method was also used in a one-pot protocol to synthesize over twenty compounds containing all-carbon quaternary centers.


 Please wait while we load your content...
Please wait while we load your content...