The hydrogen atom transfer reactivity of sulfinic acids†
Abstract
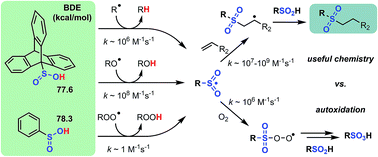
Sulfinic acids (RSO2H) have a reputation for being difficult reagents due to their facile autoxidation. Nevertheless, they have recently been employed as key reagents in a variety of useful radical chain reactions. To account for this paradox and enable further development of radical reactions employing sulfinic acids, we have characterized the thermodynamics and kinetics of their H-atom transfer reactions for the first time. The O–H bond dissociation enthalpy (BDE) of sulfinic acids was determined by radical equilibration to be ∼78 kcal mol−1; roughly halfway between the RS-H BDE in thiols (∼87 kcal mol−1) and RSO-H BDE in sulfenic acids (∼70 kcal mol−1). Regardless, RSH, RSOH and RSO2H have relatively similar inherent H-atom transfer reactivity to alkyl radicals (∼106 M−1 s−1). Counter-intuitively, the trend in rate constants with more reactive alkoxyl radicals follows the reaction energetics: ∼108 M−1 s−1 for RSO2H, midway between thiols (∼107 M−1 s−1) and sulfenic acids (∼109 M−1 s−1). Importantly, since sulfinic and sulfenic acids are very strong H-bond donors (αH2 ∼ 0.63 and 0.55, respectively), their reactivity is greatly attenuated in H-bond accepting solvents, whereas the reactivity of thiols is largely solvent-independent. Efforts to measure rate constants for the reactions of sulfinic acids with alkylperoxyl radicals were unsuccessful. Computations predict these reactions to be surprisingly slow; ∼1000-times slower than for thiols and ∼10 000 000-times slower than for sulfenic acids. On the other hand, the reaction of sulfinic acids with sulfonylperoxyl radicals – which propagate sulfinic acid autoxidation – is predicted to be almost diffusion-controlled. In fact, the rate-determining step in sulfinic acid autoxidation, and the reason they can be used for productive chemistry, is the relatively slow reaction of propagating sulfonyl radicals with O2 (∼106 M−1 s−1).


 Please wait while we load your content...
Please wait while we load your content...