Unraveling reaction networks behind the catalytic oxidation of methane with H2O2 over a mixed-metal MIL-53(Al,Fe) MOF catalyst†
Abstract
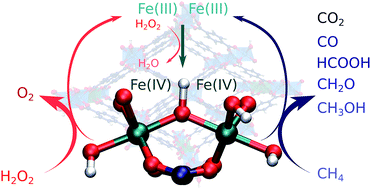
Reaction paths underlying the catalytic oxidation of methane with H2O2 over an Fe containing MIL-53(Al) metal–organic framework were studied by periodic DFT calculations. Not only the activation of methane, but the full reaction network was considered, which includes the formation of the active site, the overoxidation of methane to CO2 and the decomposition of H2O2 to H2O and O2. Calculations indicate that the activation barrier for the initial activation of the Fe sites upon reaction with H2O2 is comparable to that of the subsequent C–H activation and also of the reaction steps involved in the undesirable overoxidation processes. The pronounced selectivity of the oxidation reaction over MIL-53(Al,Fe) towards the target mono-oxygenated CH3OH and CH3OOH products is attributed to the limited coordination freedom of the Fe species encapsulated in the extended octahedral [AlO6] structure-forming chains, which effectively prevents the direct overoxidation paths prior to product desorption from the active sites. Importantly, our computational analysis reveals that the active sites for the desired methane oxidation are able to much more efficiently promote the direct catalytic H2O2 decomposition reaction, rendering thus the current combination of the active site and the reactants undesirable for the prospective methane valorization process.
- This article is part of the themed collection: 2018 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...