Abstract
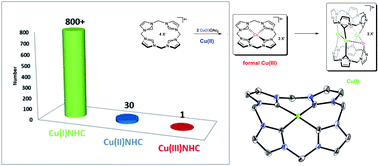
For years, Cu(III)NHCs have been proposed as active intermediates in Cu(I)NHC catalyzed reactions, yielding the desired products by reductive elimination, but until today, no one has ever reported the characterisation of such a compound. When working on the synthesis of biomimetic transition metal (NHC) complexes and their application in homogeneous catalysis, we recently found a highly unusual reactivity for Cu(II) acetate in the presence of a particular cyclic tetra(NHC) ligand. Therein, the formation of the first stable CuNHC compound, displaying Cu in the formal oxidation state +III, by simple disproportionation of Cu(II) acetate in dimethyl sulfoxide (DMSO) was observed. At elevated temperatures selective mono-oxidation of the NHC ligand occurs, even under anaerobic conditions. Acetate was identified as the origin of the oxygen atom by 18O-labelling experiments. The remarkably high stability of the title compound was furthermore proven electrochemically by cyclic voltammetry. An in-depth investigation of its reactivity revealed the involvement of four additional compounds. Three of them could be isolated and characterised by 1H/13C-NMR, single crystal XRD, mass spectrometry and elemental analysis. The fourth, a Cu(I)NHC intermediate, formed by formal reductive elimination from the Cu(NHC)3+ compound, was characterised in situ by 1H/13C-NMR and computational methods.


 Please wait while we load your content...
Please wait while we load your content...