Radical difluoromethylthiolation of aromatics enabled by visible light†
Abstract
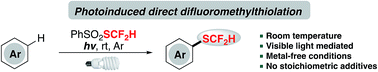
Direct introduction of a difluoromethylthio group (–SCF2H) to arenes represents an efficient route to access a valuable catalogue of organofluorines; however, to realize this transformation under metal-free and mild conditions still remains challenging and rarely reported. Herein, a metal-catalyst-free and redox-neutral innate difluoromethylthiolation method with a shelf-stable and readily available reagent, PhSO2SCF2H, under visible light irradiation is described. This light-mediated protocol successfully converts a broad spectrum of arenes and heteroarenes to difluoromethylthioethers in the absence of noble metals and stoichiometric amounts of additives.
- This article is part of the themed collection: Editor's choice - Paolo Melchiorre


 Please wait while we load your content...
Please wait while we load your content...