Rhodium catalyzed cascade cyclization featuring B–H and C–H activation: one-step construction of carborane-fused N-polyheterocycles†
Abstract
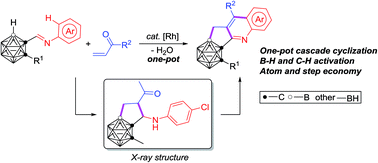
A one-pot strategy for efficient and facile synthesis of C,B-substituted carborane-fused N-polyheterocycles is reported. A rhodium catalyzed cascade cyclization of carboranyl N-arylimines with vinyl ketones enables the effective construction of three new B–C and C–C bonds in one reaction. Both carboranyl B–H and aryl C–H bonds are sequentially activated, leading to a series of previously unavailable C,B-substituted carborane-fused cyclopenta[b]quinoline derivatives, for potential applications in pharmaceuticals and materials, in a step-economical manner. The successful isolation and structural identification of a key intermediate provide solid evidence for the reaction mechanism, involving a tandem sequence of regioselective B–H activation, alkene insertion, nucleophilic cyclization, C–H activation, nucleophilic cyclization, dehydration and oxidative aromatization.


 Please wait while we load your content...
Please wait while we load your content...