Enantioselective synthesis of amines by combining photoredox and enzymatic catalysis in a cyclic reaction network†
Abstract
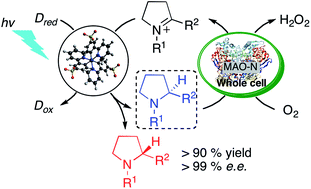
Visible light-driven reduction of imines to enantioenriched amines in aqueous solution is demonstrated for the first time. Excitation of a new water-soluble variant of the widely used [Ir(ppy)3] (ppy = 2-phenylpyridine) photosensitizer in the presence of a cyclic imine affords a highly reactive α-amino alkyl radical that is intercepted by hydrogen atom transfer (HAT) from ascorbate or thiol donors to afford the corresponding amine. The enzyme monoamine oxidase (MAO-N-9) selectively catalyzes the oxidation of one of the enantiomers to the corresponding imine. Upon combining the photoredox and biocatalytic processes under continuous photo-irradiation, enantioenriched amines are obtained in excellent yields. To the best of our knowledge, this is the first demonstration of a concurrent photoredox- and enzymatic catalysis leading to a light-driven asymmetric synthesis of amines.


 Please wait while we load your content...
Please wait while we load your content...