Structurally characterized terminal manganese(iv) oxo tris(alkoxide) complex†
Abstract
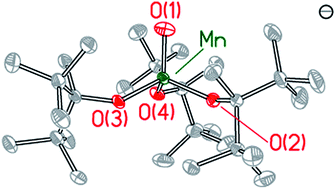
A Mn(IV) complex featuring a terminal oxo ligand, [MnIV(O)(ditox)3][K(15-C-5)2] (3; ditox = tBu2MeCO−, 15-C-5 = 15-crown-5-ether) has been isolated and structurally characterized. Treatment of the colorless precursor [MnII(ditox)3][K(15-C-5)2] (2) with iodosobenzene affords 3 as a green free-flowing powder in high yields. The X-ray crystal structure of 3 reveals a pseudotetrahedral geometry about the central Mn, which features a terminal oxo (d(Mn–Oterm = 1.628(2) Å)). EPR spectroscopy, SQUID magnetometry, and Evans method magnetic susceptibility indicate that 3 consists of a high-spin S = 3/2 Mn(IV) metal center. 3 promotes C–H bond activation by a hydrogen atom abstraction. The [MnIV(O)(ditox)3]− furnishes a model for the proposed terminal oxo of the unique manganese of the oxygen evolving complex of photosystem II.


 Please wait while we load your content...
Please wait while we load your content...