Probing the interaction between the encapsulated water molecule and the fullerene cages in H2O@C60− and H2O@C59N−
Abstract
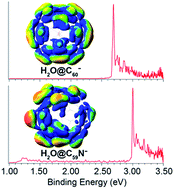
We report a high-resolution photoelectron imaging study of cryogenically-cooled H2O@C60− and H2O@C59N− endohedral fullerene anions. The electron affinity (EA) of H2O@C60 is measured to be 2.6923 ± 0.0008 eV, which is 0.0088 eV higher than the EA of C60, while the EA of H2O@C59N is measured to be 3.0058 eV ± 0.0007 eV, which is 0.0092 eV lower than the EA of C59N. The opposite shifts are found to be due to the different electrostatic interactions between the encapsulated water molecule and the fullerene cages in the two systems. There is a net coulombic attraction between the guest and host in H2O@C60−, but a repulsive interaction in H2O@C59N−. We have also observed low-frequency features in the photoelectron spectra tentatively attributed to the hindered rotational excitations of the encapsulated H2O molecule, providing further insights into the guest–host interactions in H2O@C60− and H2O@C59N−.
- This article is part of the themed collection: 2018 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...